Kybella
Diminish the Double chin
with a treatment tailored to you.
Kybella
Andra's Results
Results are represented over the course of treatment; not all treatments are shown. Number of treatments is tailored* to the amount of fat below the chin and aesthetic goals; 59% of patients received 6 KYBELLA® treatments in clinical studies.
*Multiple injections under the chin per treatment; up to 6 treatments at least 1 month apart.
Results are represented over the course of treatment; not all treatments are shown. Number of treatments is tailored* to the amount of fat below the chin and aesthetic goals; 59% of patients received 6 KYBELLA® treatments in clinical studies.
*Multiple injections under the chin per treatment; up to 6 treatments at least 1 month apart.



Unretouched photos of paid model. Individual results may vary.
Sex: F Age: 35 Weight (before/after): 142.5 lbs/144.0 lbs Total treatments: 3 Total mLs (all treatment sessions): 12.0
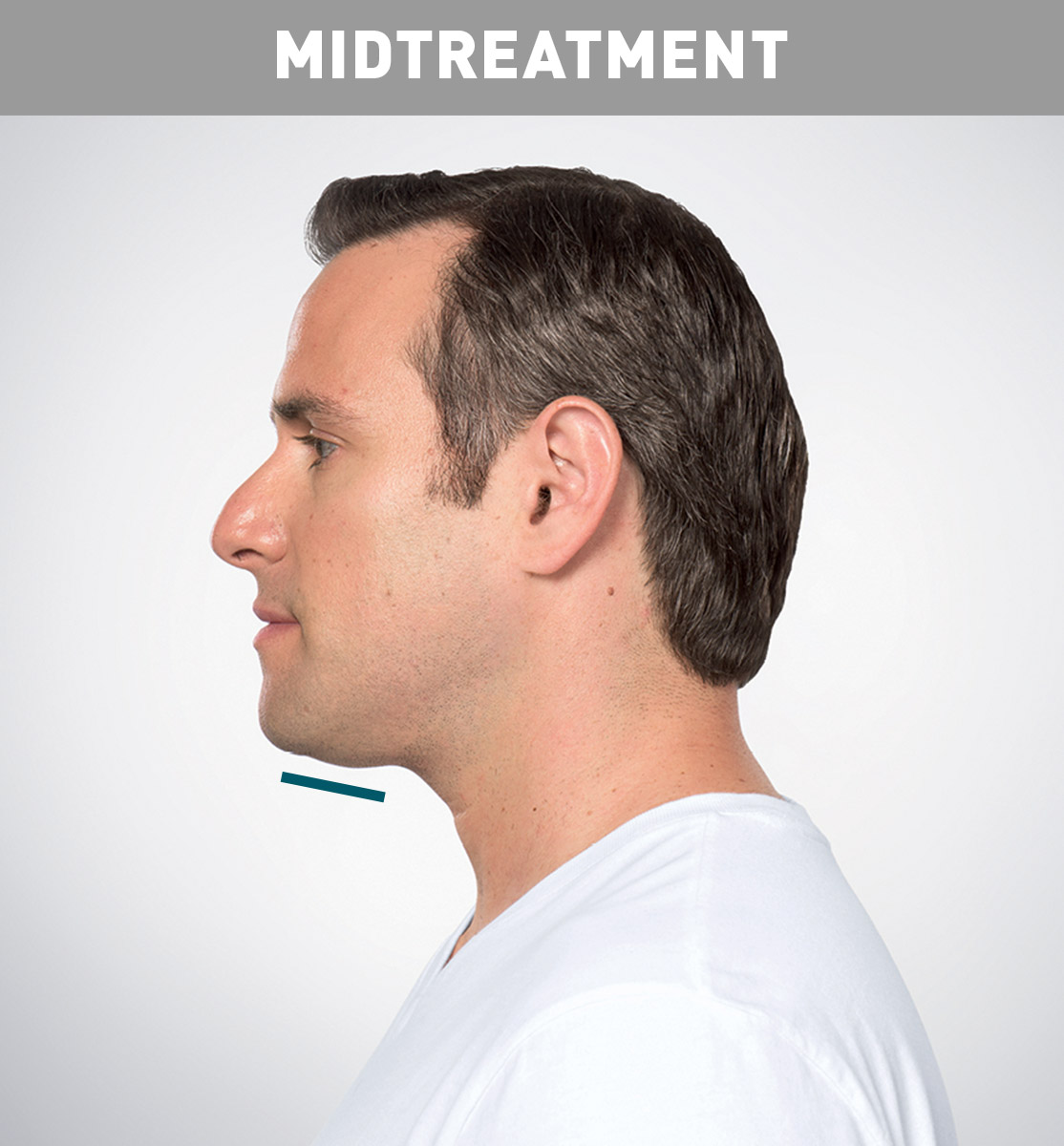
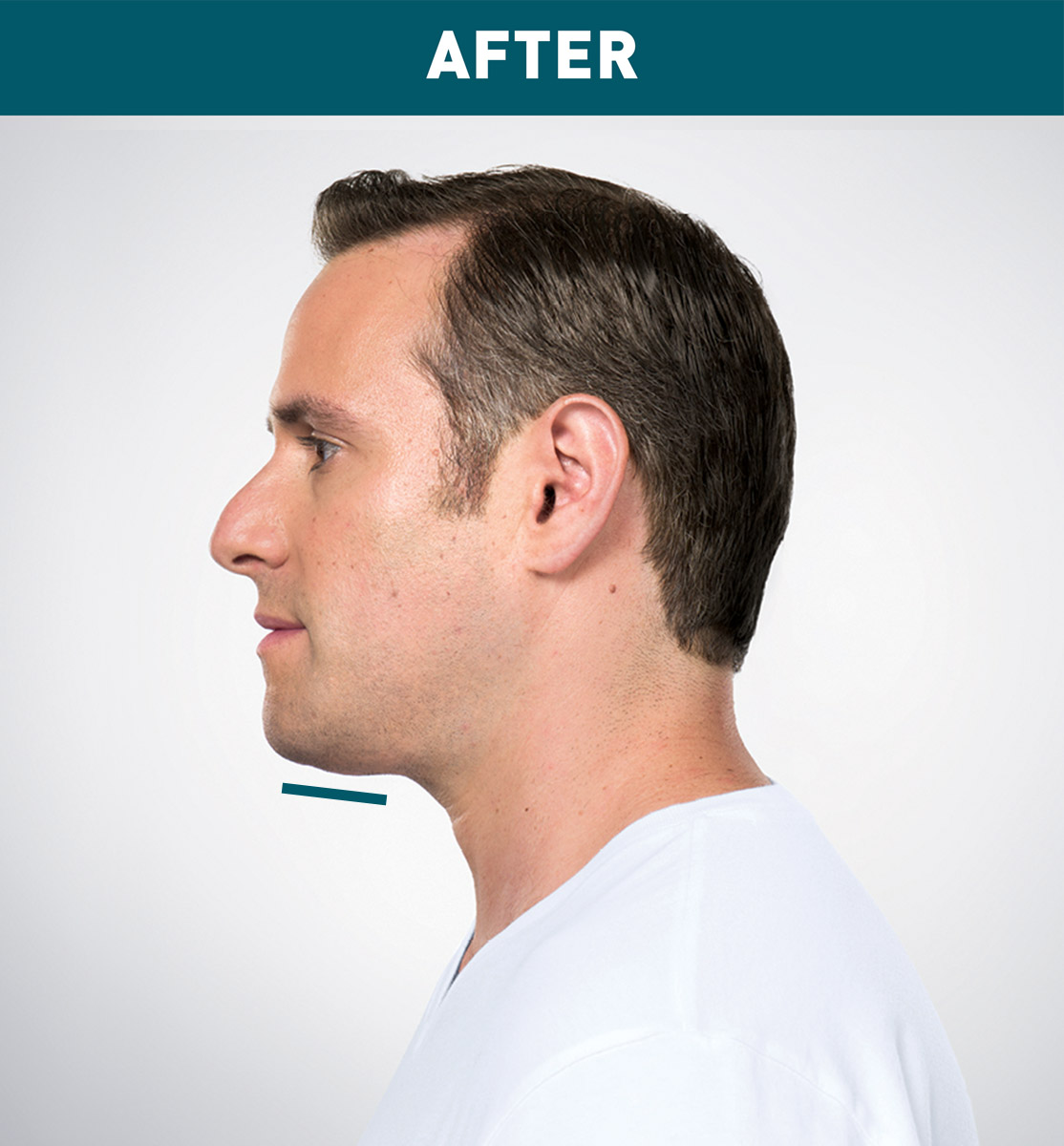
Jason's Results
Results are represented over the course of treatment; not all treatments are shown. Number of treatments is tailored* to the amount of fat below the chin and aesthetic goals; 59% of patients received 6 KYBELLA® treatments in clinical studies.
*Multiple injections under the chin per treatment; up to 6 treatments at least 1 month apart.
Results are represented over the course of treatment; not all treatments are shown. Number of treatments is tailored* to the amount of fat below the chin and aesthetic goals; 59% of patients received 6 KYBELLA® treatments in clinical studies.
*Multiple injections under the chin per treatment; up to 6 treatments at least 1 month apart.

Unretouched photos of paid model. Individual results may vary.
Sex: M Age: 36 Weight (before/after): 180.0 lbs/178.0 lbs Total treatments: 6 Total mLs (all treatment sessions): 28.0
Brabella
Patient A
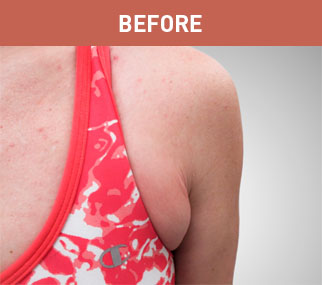
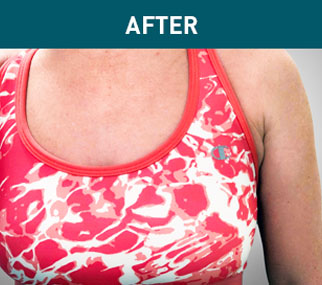
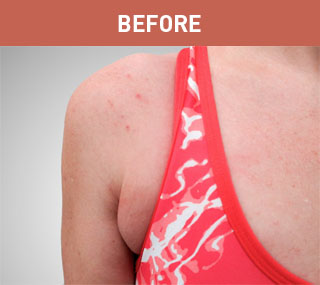
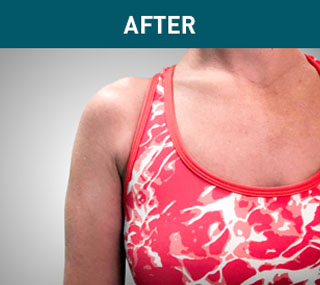
Patient B
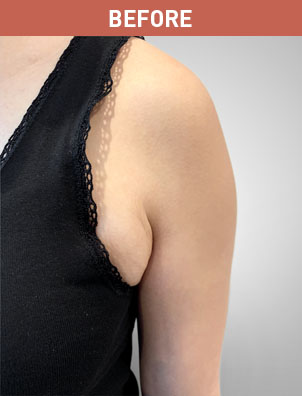

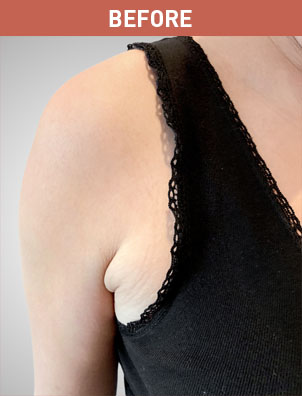




KYBELLA® (deoxycholic acid) injection 10 mg/mL Important Information
INDICATION
KYBELLA® (deoxycholic acid) injection is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults.
The safe and effective use of KYBELLA® for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
KYBELLA® is contraindicated in the presence of infection at the injection sites.
WARNINGS AND PRECAUTIONS
Marginal Mandibular Nerve Injury
Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness, were reported in 4% of subjects in the clinical trials; all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA® should not be injected into or in close proximity to the marginal mandibular branch of the facial nerve.
Dysphagia
Dysphagia occurred in 2% of subjects in the clinical trials in the setting of administration-site reactions, eg, pain, swelling, and induration of the submental area; all cases of dysphagia resolved spontaneously (range 1-81 days, median 3 days). Avoid use of KYBELLA® in patients with current or prior history of dysphagia as treatment may exacerbate the condition.
Injection-Site Hematoma/Bruising
In clinical trials, 72% of subjects treated with KYBELLA® experienced hematoma/bruising. KYBELLA® should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the treatment area may occur.
Risk of Injecting Into or in Proximity to Vulnerable Anatomic Structures
To avoid the potential of tissue damage, KYBELLA® should not be injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph nodes, and muscles.
Injection Site Alopecia
Cases of injection site alopecia have been reported with administration of KYBELLA®. Onset and duration may vary among individuals and may persist. Consider withholding subsequent treatments until resolution.
Injection Site Ulceration and Necrosis
Injections that are too superficial into the dermis may result in skin ulceration and necrosis. Cases of injection site ulceration and necrosis have been reported with administration of KYBELLA®. Do not administer KYBELLA® into affected area until complete resolution.
ADVERSE REACTIONS
The most commonly reported adverse reactions in the pivotal clinical trials were: injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and induration.
Please see KYBELLA® full Prescribing Information.